Cervical Cancer
Cervical cancer is predominantly sexually transmitted and the association between certain high risk strains of human papillomavirus (HPV) and cervical cancer is well documented. It has been shown worldwide that screening for precursors of cervical cancer by means of Papanicoloau (PAP) smears substantially reduces the risk of invasive cancer.
PAP test / PAP smear
A Pap smear, also known as Papanicoloau smear, is a microscopic examination of cells scraped from the cervix and is used to detect cancerous or pre-cancerous conditions of the cervix or other medical conditions. It was named after Dr. George N. Papanicoloau, who first described it in 1928 and since its introduction; the Pap smear has helped reduce cervical cancer incidence and mortality rates. The Pap smear is a screening method that looks for changes in the transformation zone of the cervix, which most often are caused by HPV.
Sample Collection
A PAP test should be performed during the second half of the menstrual cycle (Day 14). Sample collection usually begins with appropriate instructions to the patient. Patients opting for the test must abstain from sexual intercourse and avoid using any vaginal medication or contraceptives 48 h before sample collection. The patient is placed in lithotomy position and the cervix is visualized by means of a speculum. The smaller end of the Ayre spatula is introduced through the external Ostium of Uterus and the squamocolumnar junction is scraped by rotating the spatula to 360°. The scraping is then evenly spread onto a glass slide, which is immediately fixed using 95% ethyl alcohol and ether to avoid air drying artifacts. Doctor or nurse also performs a pelvic exam, checking the uterus, ovaries and other organs to make sure there are no problems.
who should have a PAP test?
Doctors recommend that women should begin having regular PAP tests and pelvic exams at age 21, or within three years of the first time they have sexual intercourse – whichever happens first. National guidelines recommend that after a woman has a PAP test each year for three years in a row, and test results show there are no problems, she can then get the PAP test once every 2-3 years.
PAP smear reporting
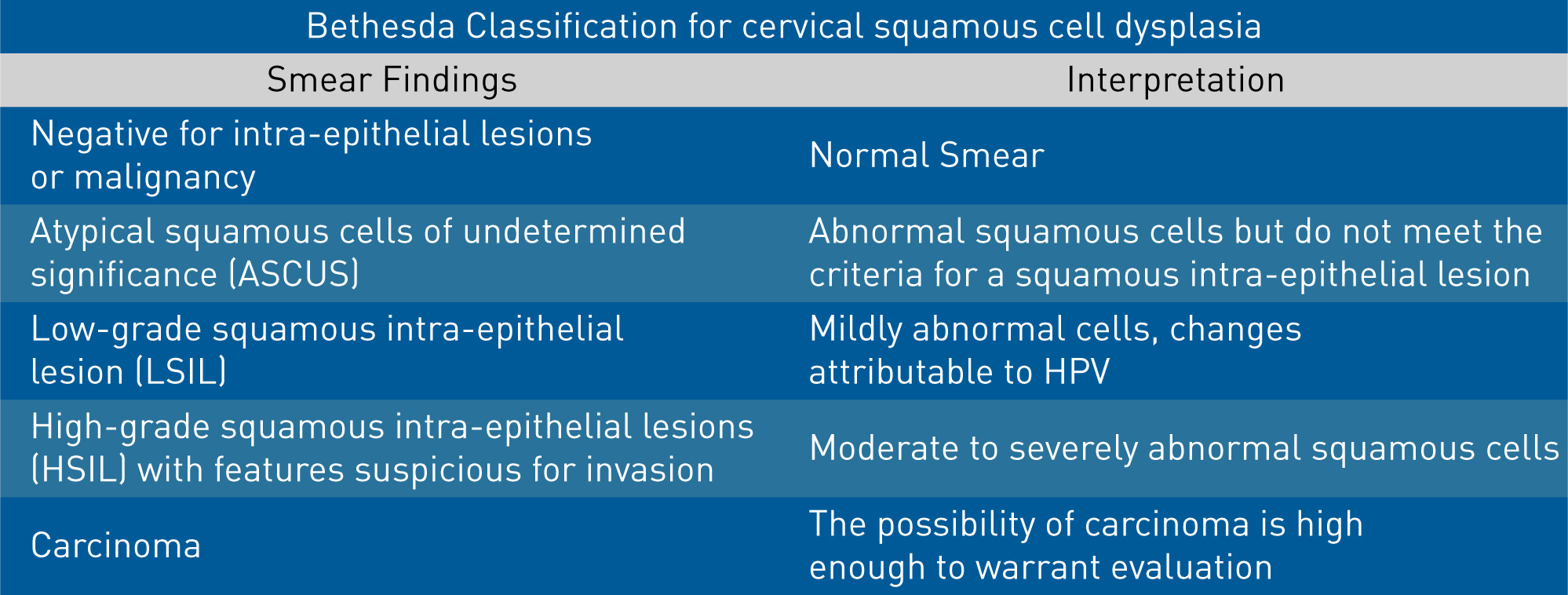
The Pap smear reporting classification has evolved and been refined over time. The current reporting system is the Bethesda system, which was introduced in 1988 and later updated again in 1999.
Limitations of conventional PAP smear
- False-negative results as high as 20-30% have been reported, which occurred due to clumping of cells when the cells are not uniformly spread on the glass slide.
- Sometimes, other contents of the cervical specimen such as blood, bacteria and yeasts contaminate the sample and prevent the detection of abnormal cells.
- If exposed to air for too long before being fixed on the slide, cervical cells can become distorted.
- Human error is probably the primary threat to accu rate interpretation. An average Pap smear slide contains 50,000-300,000 cells that must be examined and if the sample contains only a few abnormal cells within a crowded background of healthy cells, the abnormal cells may be missed.
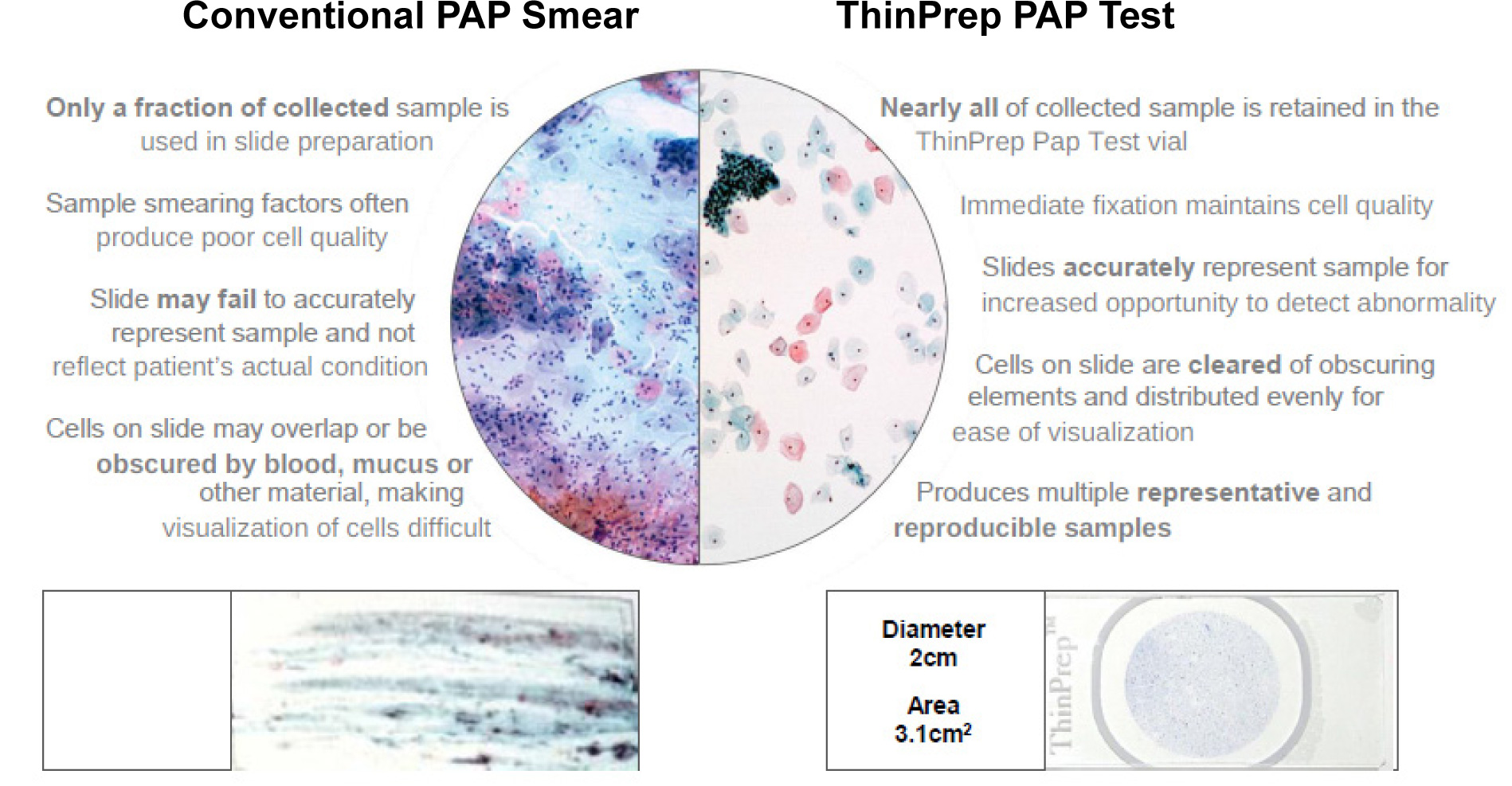
New technique – Liquid-Based Cytology (LBC)
A conventional Pap smear has a sensitivity ranging from 47 to 62% and a specificity of 60-90%. In order to minimize the number of false-negative results, Liquid Based Cytology is now the preferred method of sample collection where a cervical brush is used to collect the specimen, which provides almost twice as many epithelial cells. The samples are collected directly in a preservative solution and slides are prepared meticulously avoiding any uneven manual smearing and thus reducing human error while interpretation. LBC has got a higher sensitivity and specificity than Pap smear as the cellular structure is better preserved because the cells are fixed immediately
Hologic ThinPrep (LBC)
Trivitron, on a global mission of making healthcare affordable and accessible lays emphasis on providing cost-effective solution for cervical cancer screening. As an extension to this commitment, Trivitron being an authorized distributor of Hologic Inc, a US based leading medical technology company primarily focused on women’s health, brings a range of diagnostic equipment and technologies.
Hologic ThinPrep® 2000 System is intended as a replacement for the conventional method of Pap smear preparation for use in screening for the presence of atypical cells, cervical cancer, or its precursor lesions (Low-grade Squamous Intraepithelial Lesions, High-grade Squamous Intraepithelial Lesions), as well as all other cytologic categories as defined by The Bethesda System for Reporting Cervical/Vaginal Cytologic Diagnoses. ThinPrep 2000 with its small footprint and portability features fits into any laboratory with a work load of minimum 15 samples per day.